Free Negative Hiv Test PDF Form
Understanding the intricacies of the Negative HIV Test form is crucial for individuals undergoing HIV testing. This comprehensive document, typically issued by health agencies, meticulously records the details of the HIV testing process, encompassing not only the client's personal information such as name, date of birth, sex, and race but also the specific location where the test was conducted. The heart of the form lies in the declaration of the test result—clearly indicating whether it is reactive or non-reactive. Additionally, it includes provisions for documenting follow-up appointments, ensuring a continuum of care for the client. The form also serves as a record-keeping tool, with sections dedicated to recording test site information, the Clinical Laboratory Improvement Amendments (CLIA) number, and even the storage conditions of the HIV test devices, ensuring the accuracy and reliability of the test results. Signatures from both the client and the counselor are requisite, validating the authenticity of the test procedure and results. Furthermore, the form outlines a structured log for the HIV test results, including data on specimen collection, test execution, and the review process, all of which are instrumental in maintaining the quality and integrity of HIV testing services.
Sample - Negative Hiv Test Form






File Specs
| Fact Name | Description |
|---|---|
| Form Usage | This form is used to document the result of a rapid HIV test, including client details, test results, and follow-up appointment information. |
| CLIA Compliance | The form requires the agency's CLIA (Clinical Laboratory Improvement Amendments) number, ensuring the test is performed in a compliant facility. |
| Test Result Options | HIV Antibody Screening Test Results can be marked as Reactive, Negative/Non-Reactive, providing clear outcomes of the testing process. |
| Follow-Up Appointments | Scheduling information for follow-up appointments is included, emphasizing the importance of further action after initial testing. |
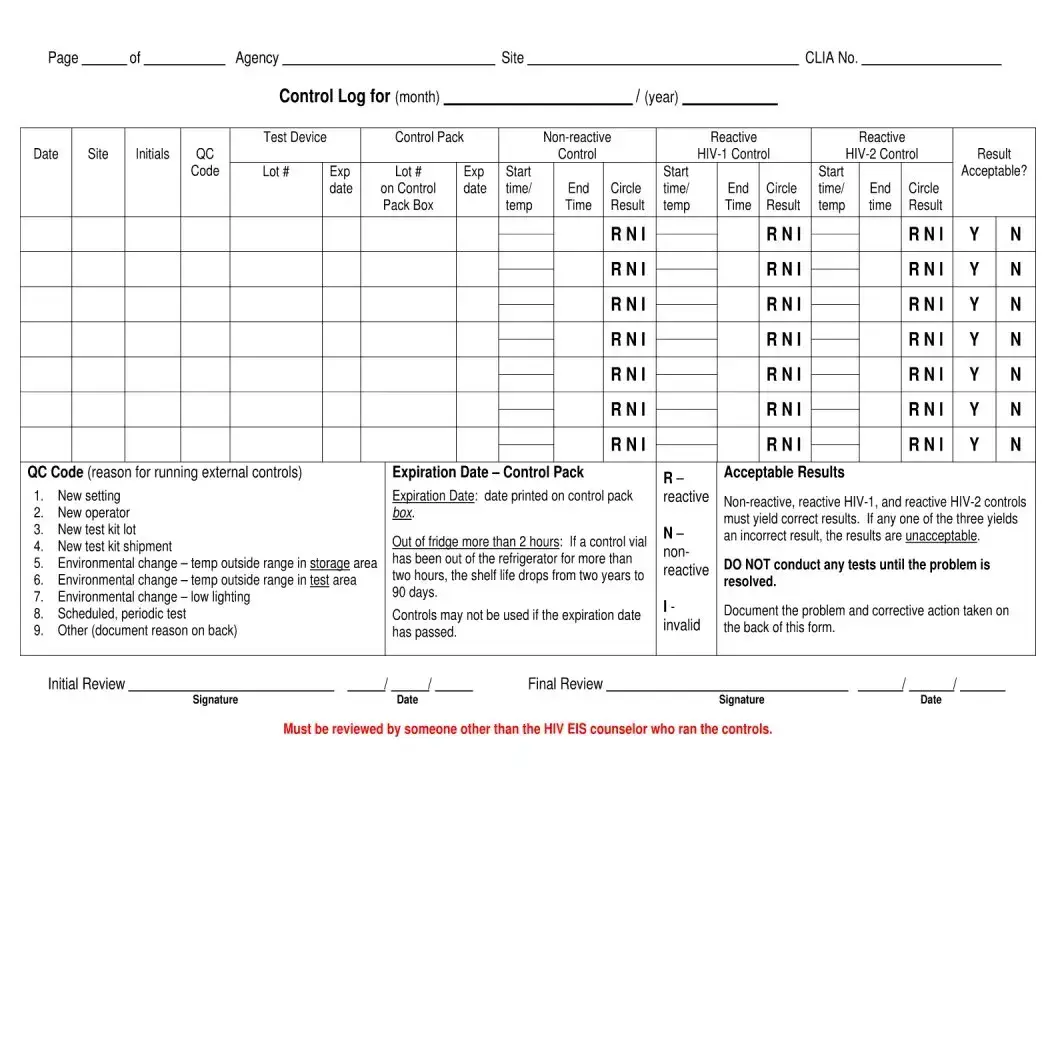
| Temperature Logs | There are specific logs for recording the storage temperatures of rapid HIV test devices and controls to ensure test integrity. |
| Governing Laws | While the form follows federal guidelines under CLIA, state-specific health regulations may further govern its use and requirements. |
| Signature Requirement | Both the client and counselor must sign the form, confirming that the test was conducted and results were communicated properly. |
Steps to Filling Out Negative Hiv Test
Filling out a Negative HIV Test form is a straightforward process that requires attention to detail. This document plays a crucial role in the reporting and management of HIV test results. Follow the steps below carefully to ensure that all the necessary information is accurately captured. This helps in maintaining the integrity of the testing process, providing clear communication between the agency and the client, and ensuring the client receives appropriate follow-up care if necessary.
- Start by entering the Agency Name, Agency Address, Agency Phone Number, and CLIA Number at the top of the form. This identifies the testing facility.
- Enter the Client Name, ensuring it is spelled correctly to avoid any confusion or misidentification.
- Fill in the Date of Birth of the client, using the format MM/DD/YYYY to maintain consistency.
- Specify the Date the test was conducted. Current dates ensure the results are timely and relevant.
- Indicate the client's Sex and Race to maintain records for demographic purposes.
- List the Testing Location. This helps in tracking where the test was performed, essential for any follow-up or queries.
- Record the HIV Antibody Screening Test Result. Mark as either Negative/Non-Reactive or Reactive, based on the outcome of the test.
- Schedule a Follow-Up Appointment by entering the date, time, and location. Even if the test is negative, follow-up can be crucial for ongoing health monitoring and support.
- Ensure the Client Signature is obtained, which confirms that the client has received and acknowledged their test result.
- Complete the process with the Counselor Signature. This verifies that the counselor has correctly administered the test and delivered the results to the client.
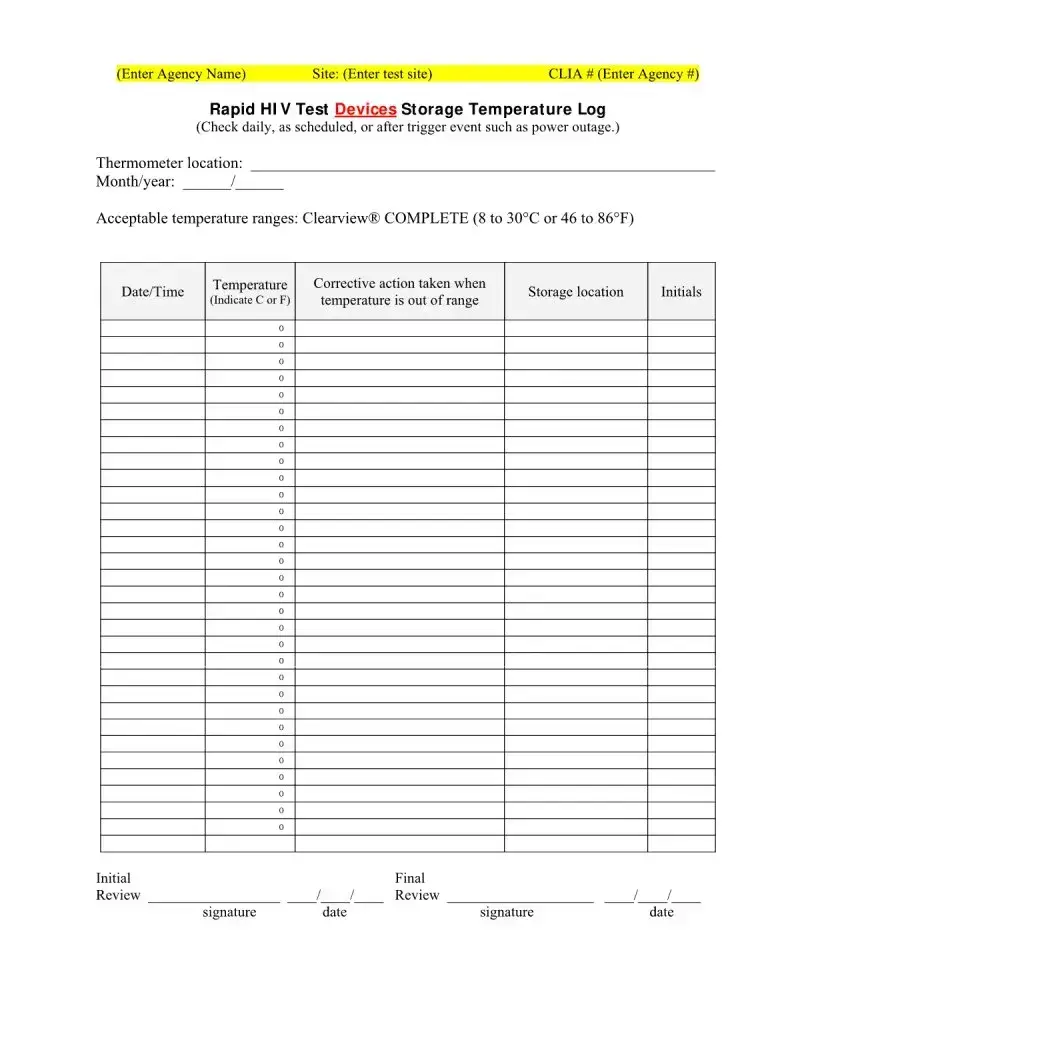
- For the temperature log sections, accurately record the daily checks or checks performed after a trigger event, such as a power outage, for both the Rapid HIV Test Devices Storage Temperature Log and the Rapid HIV Test Control Storage Temperature Log. These sections are critical for ensuring that the tests are stored within the correct temperature ranges, thus maintaining their integrity and accuracy.
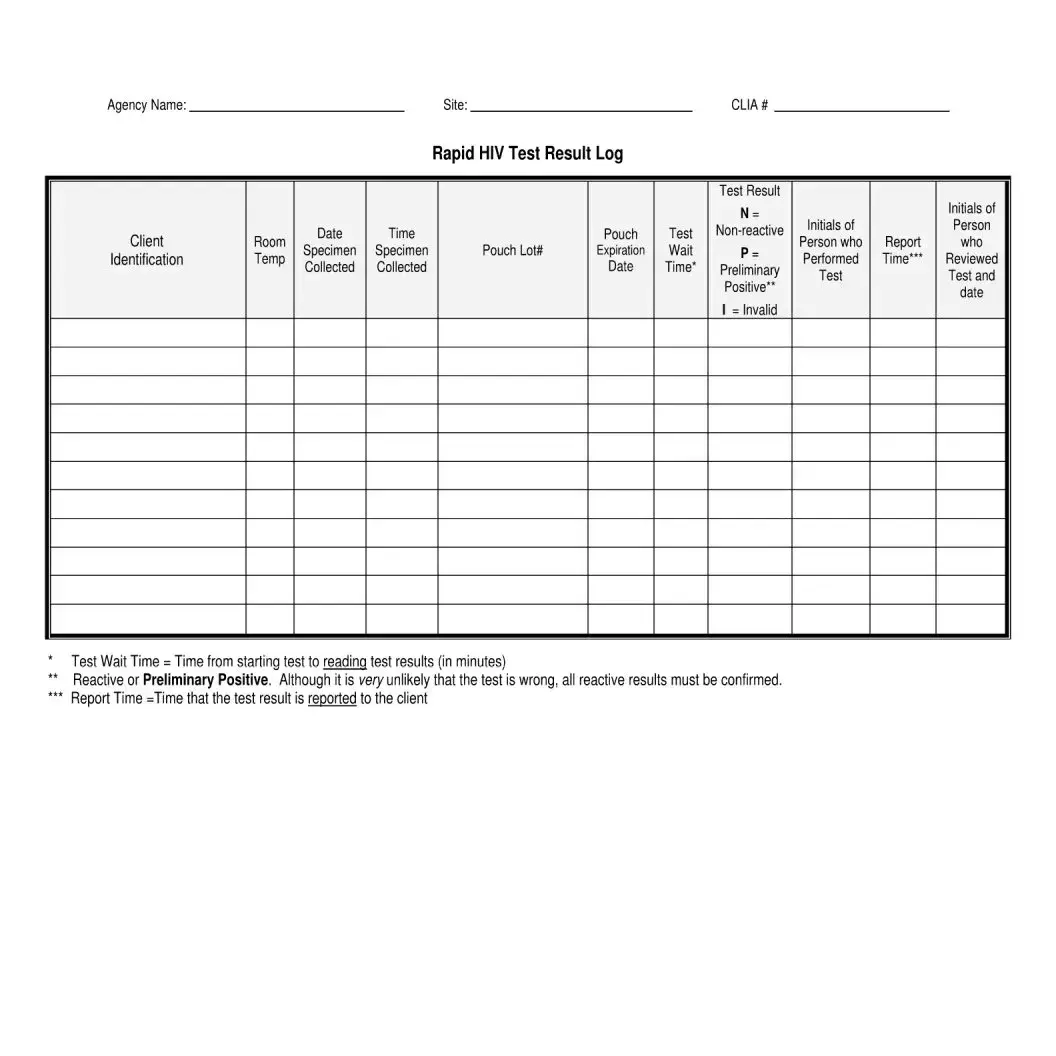
- Lastly, use the Rapid HIV Test Result Log to document each test conducted, including details such as room temperature, date and time the specimen was collected, pouch lot number, expiration date, test wait time, and initials of the client and the person who performed the test. Record the test result as N for non-reactive, P for preliminary positive, or mark it as invalid if the test did not perform as expected.
Upon completion, review the form to ensure all information is correct and legible. Proper documentation and careful attention to the steps outlined ensure the effective communication of HIV test results and support the health and well-being of clients.
Discover More on Negative Hiv Test
What is a Negative HIV Test form?
A Negative HIV Test form is a document provided by healthcare agencies or testing sites that records the result of an HIV test. It includes information such as the client's name, date of birth, race, sex, testing location, the outcome of the HIV antibody screening test, and details on any follow-up appointments. This form also verifies that the test performed was either reactive or non-reactive/negative.
What does a "non-reactive" test result mean?
A "non-reactive" test result, also marked as negative, means that no HIV antibodies were detected in the test sample. It suggests that the individual tested does not have HIV at the time of the test. However, it's important to remember that there is a window period during which the virus may not be detectable. Follow-up testing may be recommended.
Why does the form include a section for follow-up appointments?
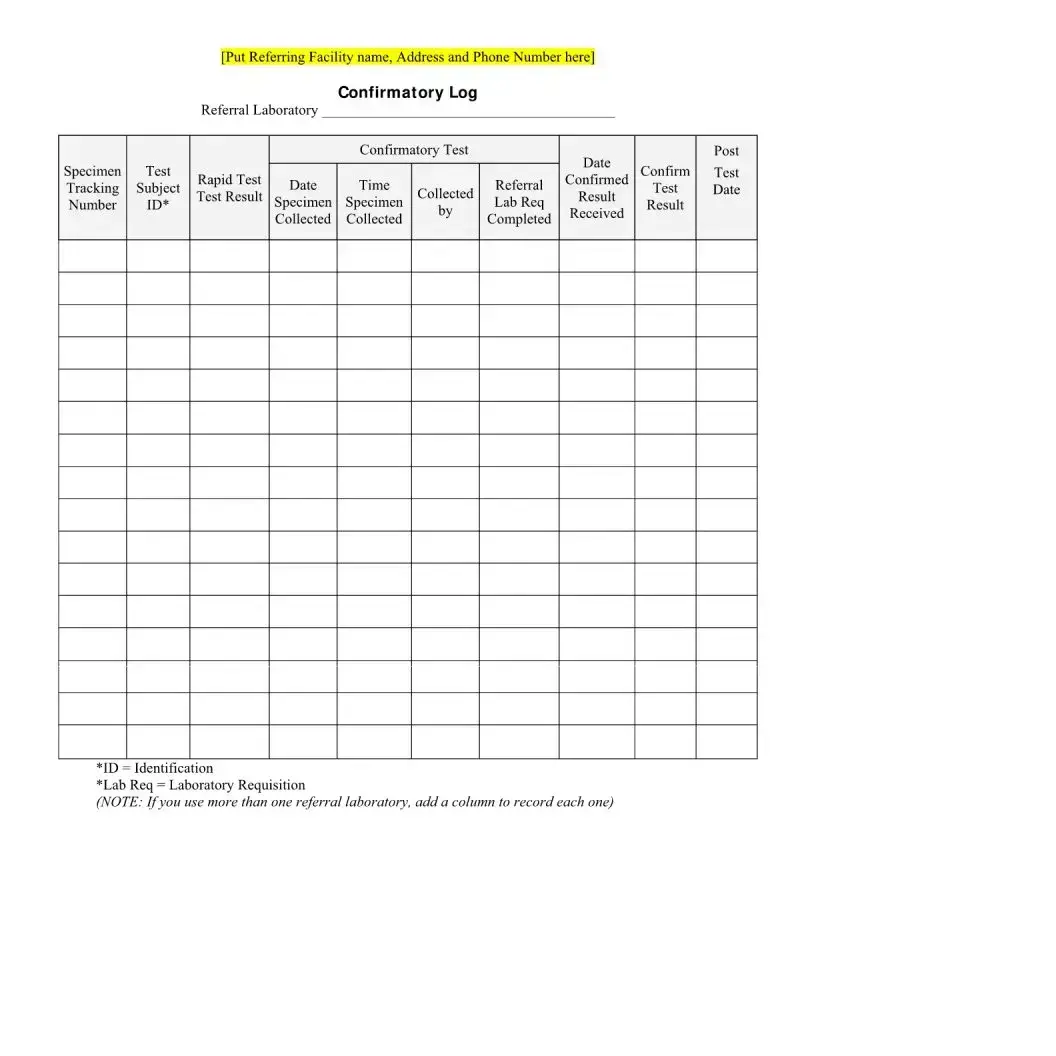
The section for follow-up appointments is included to ensure that individuals receive the necessary support and further testing if required. For those with a reactive (preliminary positive) result, confirmatory testing is crucial for accurate diagnosis. Follow-up appointments also provide an opportunity for counseling and to discuss preventive measures.
What is the significance of storing rapid HIV test devices at specific temperatures?
Rapid HIV test devices must be stored within certain temperature ranges to ensure their accuracy and reliability. If the devices are stored at temperatures outside these ranges, it may compromise the test results. The form records daily temperature checks, corrective actions taken if temperatures deviate, and storage details to maintain the integrity of the testing process.
How is client confidentiality protected with this form?
Client confidentiality is maintained by securely storing these forms and limiting access to them. Personal identifiers and test results are sensitive information, and agencies are responsible for ensuring that only authorized personnel can view or handle these forms. Additionally, client consent is required for any disclosure of this information.
What are CLIA numbers, and why are they included on the form?
CLIA numbers reference the Clinical Laboratory Improvement Amendments certification. This certification indicates that the testing site adheres to federal standards for quality lab testing. Including the CLIA number on the form validates the legitimacy and accuracy of the test results.
What does it mean if a test result is marked as "Invalid"?
A test result marked as "Invalid" indicates that the test did not produce a definitive result. This can occur for a variety of reasons, such as improper sample collection or a malfunction of the test device. In such cases, the test is typically repeated to obtain accurate results.
Common mistakes
Filling out forms is a routine part of accessing services, but when it comes to health-related documents like the Negative HIV Test form, accuracy is paramount. Making mistakes can lead to confusion, delays in receiving information, and in certain cases, incorrect reporting of a person’s health status. Here are some common mistakes people should avoid:
- Not Checking the Accuracy of Personal Information: Every detail, from the client name to the date of birth, must be double-checked for accuracy. Incorrect personal information can result in misfiled results or other administrative issues.
- Skipping Sections: Omitting information, such as the testing location or the date, may seem harmless but can lead to significant confusion or delays in processing the form.
- Misunderstanding Test Results: Marking the wrong box in the HIV Antibody Screening Test Result section could convey the opposite of what is true. It's crucial to understand the difference between 'Reactive' and 'Negative/Non-Reactive' responses.
- Forgetting to Schedule the Follow-Up Appointment: The follow-up appointment is a critical next step for clients, regardless of the test outcome. If this section is left blank, it could interfere with necessary future care.
- Incomplete Signatures: Both the client and the counselor must sign the form. Unfinished signatures may question the validity of the form, causing administrative holdups.
- Overlooking Temperature Logs for Test Devices: For agencies managing the test, maintaining and accurately filling out the test devices’ storage temperature logs is critical. Failing to do so can compromise the test results.
To avoid these mistakes, every individual involved, from the client to the health personnel, should handle the Negative HIV Test form with great attention to detail. Here are some steps to ensure the form is correctly filled out:
- Review each section carefully before moving on to the next.
- Make sure all required fields are populated with the correct information.
- Understand the meanings of all possible test outcomes.
- Confirm follow-up appointments and record them on the form.
- Ensure all signatures are complete before submitting the form.
- Regularly check and log the storage temperatures of test devices as required.
By avoiding these common mistakes, clients and health care providers can work together to ensure that HIV testing is conducted smoothly and accurately, which is crucial for proper care and treatment.
Documents used along the form
When managing and documenting the process surrounding HIV testing, several crucial forms and documents complement the Negative HIV Test Form. These documents ensure a comprehensive and responsible approach to testing, results handling, and patient care. Each document serves a specific purpose in the continuum of care, from test administration to follow-up consultations.
- Consent for HIV Testing Form: This document is vital as it records the patient's consent to undergo HIV testing. It includes an acknowledgment of understanding the test purpose, potential outcomes, and the confidentiality of results. The form is a safeguard for both the patient and the healthcare provider, ensuring informed consent is obtained before testing.
- Pre-Test Counseling Form: Pre-test counseling is an essential step in the HIV testing process. This form documents that the patient received counseling before the test, covering the implications of possible test results, information on HIV transmission, and prevention methods. It ensures that the patient makes an informed decision about testing and understands the subsequent steps based on the test outcome.
- Post-Test Counseling Form: Following the delivery of test results, post-test counseling provides essential support and information. This document outlines the counseling provided after the test, including interpreting the results, discussing next steps in case of a positive result (e.g., further testing for confirmation, treatment options), or reinforcing prevention methods if the result is negative.
- Referral Form for Confirmatory Testing: In cases where the initial HIV test result is reactive (preliminary positive), this form facilitates the referral process for confirmatory testing. It includes details on the referred healthcare facility, specific tests recommended, and any preliminary results that inform the need for further testing.
- Medical Follow-Up Appointment Schedule: This document is used to organize and track follow-up appointments, whether for further diagnostic testing, treatment initiation, or ongoing care in case of a positive diagnosis. It serves as a schedule for the patient, ensuring they receive proper medical support and monitoring over time.
Together, these documents form a comprehensive framework supporting the HIV testing process. They ensure that patients are well-informed, consent to testing, understand their results, and receive appropriate care and counseling at every step. This holistic approach not only facilitates effective healthcare delivery but also respects and safeguards patient rights and well-being.
Similar forms
A Medical Test Result Form shares similarities with a Negative HIV Test form as it also typically includes personal identification details, test results, and specifies if further action is required (such as a follow-up appointment). Both forms serve as official records of an individual's health status as determined by specific tests.
The Consent Form for Medical Treatment is similar in that it requires the client's signature, much like the Negative HIV Test form. Though one is for documenting consent and the other for documenting test results, both are crucial in health services for verifying that the patient agrees to the procedure or acknowledges the results.
A Vaccine Administration Record (VAR) is comparable because it too collects client identification information, details about the medical intervention (in this case, vaccination), and includes follow-up information. Similarly, it is used to track a patient's health intervention over time and ensure proper health management.
The Laboratory Test Request Form also bears resemblance, primarily in its function of recording critical test-related information like patient details, test type, and date. While one form is used to request tests and the other to report results, both are integral in the healthcare process, ensuring that correct and necessary tests are performed and results accurately communicated.
Dos and Don'ts
Filling out a Negative HIV Test form is a crucial process that requires attention to detail and accuracy. Here are some dos and don'ts to consider:
- Do double-check the client's personal information (name, date of birth, and race) for accuracy. This ensures that the test results are correctly attributed and prevents any mix-up in records.
- Do accurately enter the date and location of the testing to maintain a clear record of when and where the test was administered. This information is essential for any follow-up appointments or consultations.
- Do carefully mark the HIV Antibody Screening Test Result section to clearly indicate if the result is 'Reactive' or 'Negative/Non-Reactive.' Precise reporting is crucial for appropriate follow-up actions and counseling.
- Do ensure that both the client and the counselor sign the form. These signatures validate the test process and confirm that both parties have acknowledged the test results.
- Don't forget to enter the agency's name, address, phone number, and CLIA number at the beginning of the form. These details are vital for record-keeping and validation purposes.
- Don't overlook the importance of documenting the follow-up appointment (date, time, location) if applicable. Proper documentation helps in ensuring continuity of care and support for the client.
- Don't leave any section blank unless it is explicitly stated as optional or not applicable. Incomplete forms may lead to misunderstandings or administrative issues later on.
- Don't rush through the process without verifying the storage temperature logs for the test devices, if applicable. Proper storage conditions are critical to the accuracy of the test results.
Approaching the Negative HIV Test form with attention and care not only ensures the accuracy of the information recorded but also upholds the integrity of the testing process. Following these guidelines can aid in providing reliable and efficient services to clients.
Misconceptions
When it comes to understanding the intricacies of a Negative HIV Test form, several misconceptions may arise. These forms are not only crucial for the individual being tested but also serve as an essential tool in the healthcare provider's arsenal for managing and preventing the spread of HIV. Let's address five common misunderstandings:
- The meaning of "Negative/Non-Reactive". A common misconception is that a "Negative" or "Non-Reactive" result on the HIV Antibody Screening Test means the individual is immune to HIV. In reality, this result indicates that the HIV antibodies were not detected in the person's system at the time of the test. It's crucial to remember that there is a "window period" during which the body has not yet produced enough antibodies to be detected by the test. Therefore, if exposure to the virus occurred recently before the test, a follow-up test is recommended after the window period.
- The importance of follow-up appointments. Some people believe that if their result is Negative/Non-Reactive, follow-up appointments are unnecessary. However, these appointments are critical, especially for those who might have been in their window period during the initial test. Follow-up appointments ensure accurate monitoring and support, providing an opportunity for re-testing and accessing preventive measures if needed.
- Interpreting "Reactive" results. Another misunderstanding is about what it means when a result is marked "Reactive." Some might jump to conclusions, believing they are HIV positive. However, a "Reactive" result indicates preliminary positive, necessitating further confirmatory tests. It's essential to understand that the initial test is a screening tool, and confirmatory tests are needed to establish an accurate diagnosis.
- Role of the storage temperature logs. The necessity of maintaining specific storage temperatures for the HIV test devices and controls might seem like a minor detail, but it's a critical aspect often underestimated. The misconception here is that these logs are just administrative paperwork. In truth, incorrect storage can compromise the test results, leading to potentially false negatives or positives. These temperature logs ensure the integrity of the testing process, guaranteeing reliable results.
- Client and Counselor Signatures. Lastly, there's a misunderstanding regarding the significance of client and counselor signatures on the form. Some may view this as a mere formality. However, these signatures are a vital part of the consent and counseling process, ensuring that clients are informed about the test, its implications, and have agreed to proceed. Additionally, the counselor's signature represents a commitment to ethical standards and confidentiality, reinforcing the trust in the client-provider relationship.
Addressing these misconceptions is key to ensuring that both individuals and healthcare providers understand the implications of the HIV test results accurately and can take appropriate actions based on them. Education and communication play indispensable roles in demystifying these aspects, contributing to more effective prevention and treatment of HIV.
Key takeaways
Understanding the Negative HIV Test form is crucial for ensuring accurate documentation and client care. Here are several key takeaways for correctly filling out and utilizing this form:
- Accuracy of Information: It’s imperative to enter all client details accurately, including name, date of birth, race, and testing location. Any errors can lead to misidentification or miscommunication.
- CLIA Number: The Clinical Laboratory Improvement Amendments (CLIA) number associated with the agency must be clearly stated. This number certifies that the testing site meets the standards for quality laboratory testing.
- HIV Test Results: The form distinctly categorizes results as either “Reactive” or “Negative/Non-Reactive”. Proper marking in the designated area is crucial for clear communication of test outcomes.
- Follow-Up Appointments: Details about follow-up appointments, including date, time, and location, must be explicitly specified for continuous client care.
- Signatures: Signatures from both the client and the counselor documenting the test result are mandatory, serving as a verification of the information provided and the test procedure.
- Rapid HIV Test Device Storage: The form requires a log of storage temperatures for the test devices. Maintaining them within the acceptable range is vital for test accuracy.
- Temperature Control Log: Recording any corrective actions taken when storage temperatures deviate from acceptable ranges helps ensure the quality and reliability of testing devices.
- Comprehensive Record Keeping: The form also stipulates logging of detailed test information, including date and time of specimen collection, test result, individual performing the test, and review details. This ensures a thorough tracking and reporting system.
Proper utilization of the Negative HIV Test form not only streamlines the process of recording test outcomes but also enhances the overall quality of care provided to the client. Strict adherence to the guidelines mentioned ensures the integrity and accuracy of the testing process.
Common PDF Forms
Free Printable Puppy Health Guarantee Template - A provision for the examination of the puppy by the buyer's veterinarian within 72 business hours after delivery or pickup is included to confirm the puppy's health status, illustrating the commitment to the puppy’s well-being.
Puppy Health Record - Aid in keeping your puppy healthy by outlining a clear schedule for vaccinations, de-worming, and other preventive measures.
